Loftair Inhaler is a combination drug formulated for the long-term maintenance treatment of Chronic Obstructive Pulmonary Disease (COPD), including emphysema and chronic bronchitis. This inhaler contains two active pharmaceutical ingredients: Indacaterol, an ultra long-acting beta2-adrenergic agonist (ultra-LABA) that relaxes and widens the airways, and Glycopyrrolate (also known as Glycopyrronium), a long-acting muscarinic antagonist (LAMA) that reduces secretions in the airways and prevents airway muscle tightening.
Key Features
| About | |
|---|---|
| Drug Class | Bronchodilators |
| Subclass | Long-acting beta2-adrenergic agonist (LABA) and anticholinergic combination |
| Product Details | |
|---|---|
| Composition | Active ingredients:
|
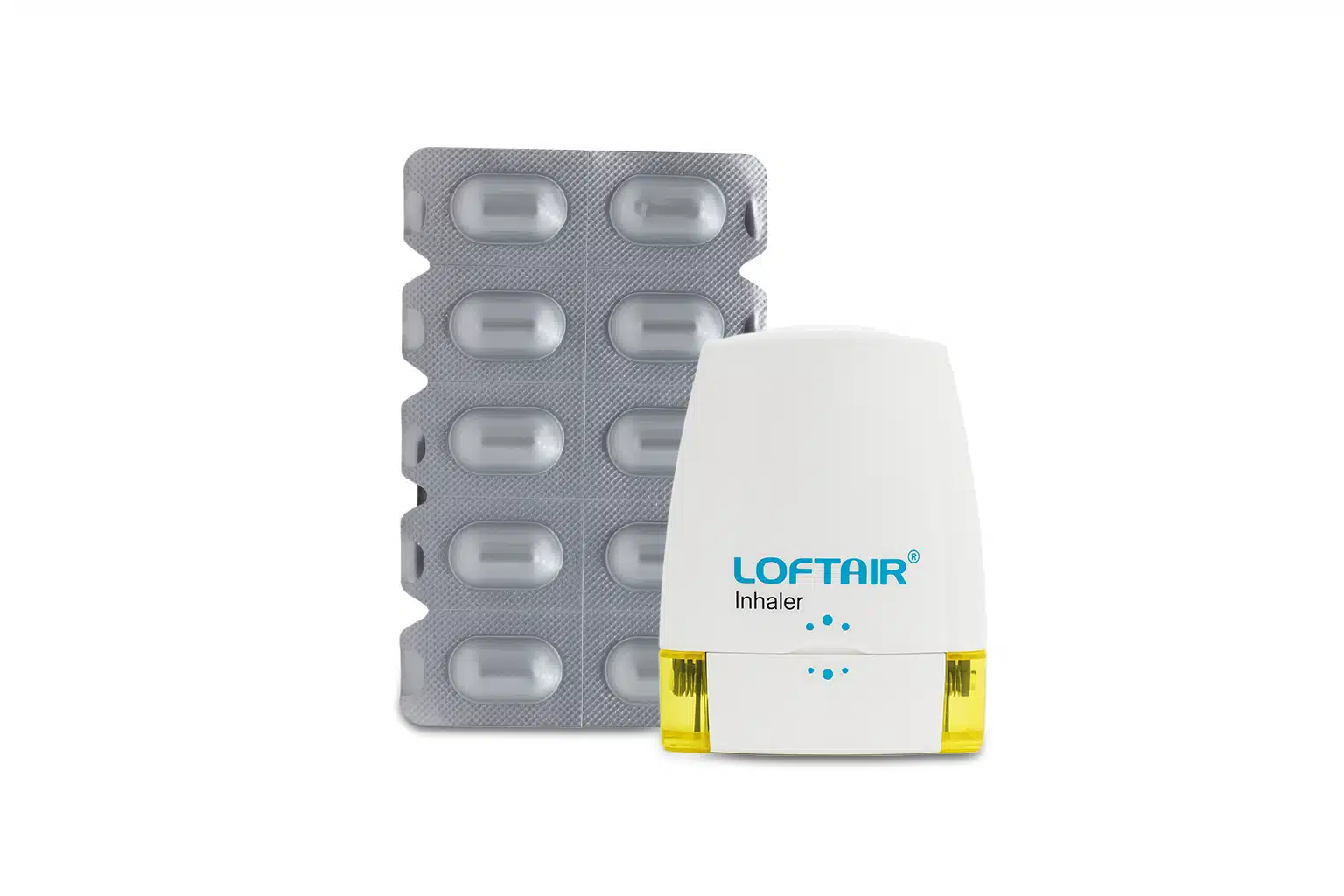
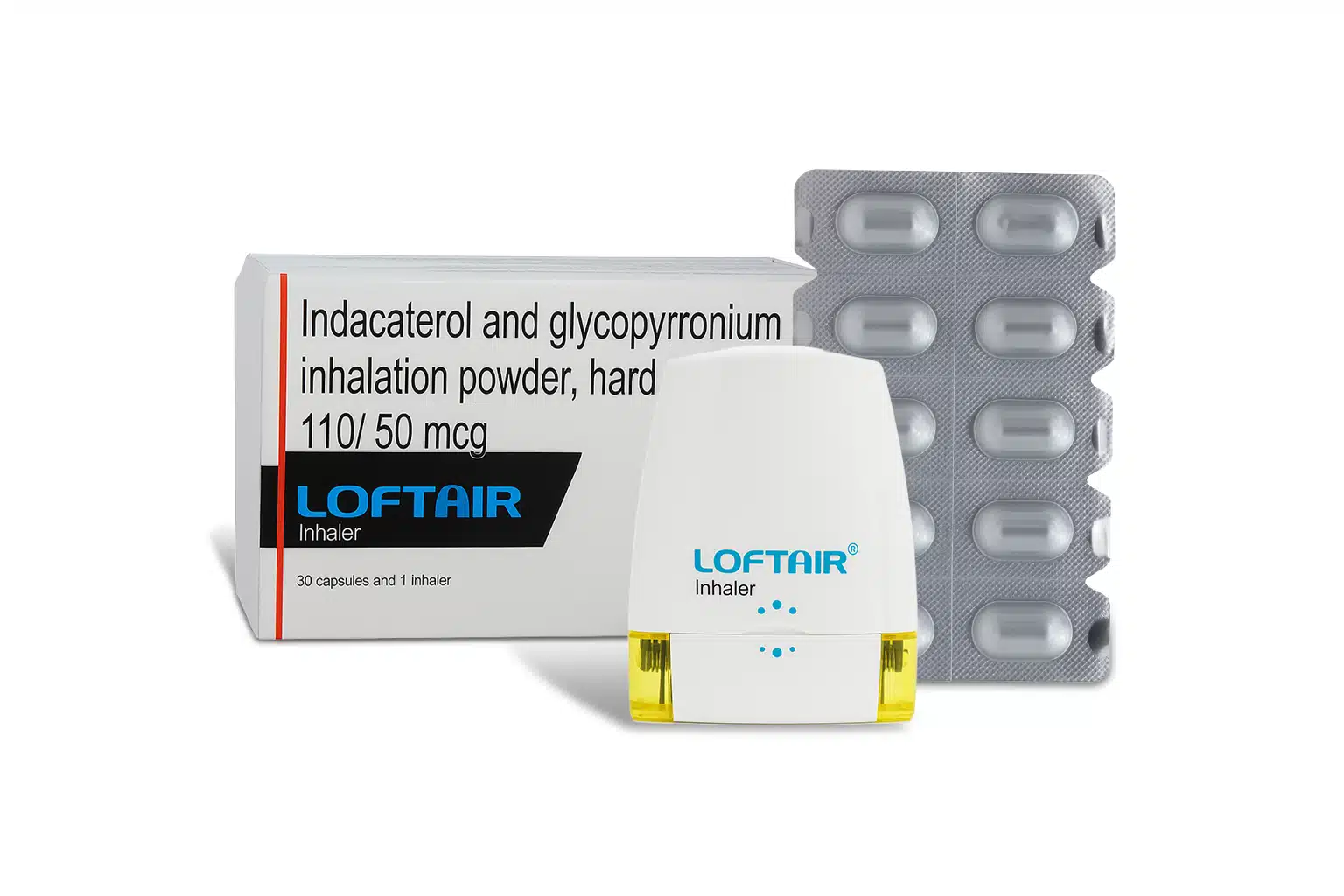
| Packaging Type | Inhaler |
| Pack Size | 30 capsules |
| Dosage | 1 inhalation twice a day, as prescribed by a healthcare provider |
| Therapeutic class | COPD Management |
| Action Class | Bronchodilator |
| Chemical class | Indacaterol (ultra-LABA), Glycopyrrolate (LAMA) |
| Manufacturer | Lupin Ltd |
| Shelf Life | 2-3 years from the date of manufacturing |
| Usages | Treatment of Chronic Obstructive Pulmonary Disease (COPD) |
| Country of Origin | India |
| Storage | Store below 30°C in a cool and dry place, protected from direct sunlight, moisture, and heat. |
How does Loftair Inhaler work?
- Relaxing and widening the airways: Indacaterol maleate, an ultra long-acting beta2-agonist, stimulates beta2-adrenergic receptors in the respiratory tract smooth muscles, causing them to relax. This process activates the adenyl cyclase enzyme which catalyzes the conversion of ATP to cyclic AMP, resulting in bronchodilation that allows easier airflow through the airways.
- Reducing airway secretions and preventing constriction: Glycopyrrolate, a long-acting muscarinic antagonist, competitively binds to muscarinic acetylcholine receptors and blocks the action of acetylcholine. This prevents airway muscles from tightening and reduces excessive secretions from the pharyngeal, tracheal, and bronchial regions.
- Improving breathing and lung function: The combined effects of these two ingredients provide rapid and sustained bronchodilation, typically starting within 5 minutes of inhalation. This dual-action mechanism offers more significant and prolonged improvements in lung function compared to using either component alone, helping to relieve breathlessness, reduce COPD exacerbations, and improve quality of life.
Dosage
- 1 capsule inhaled once daily at the same time each day, or as directed by your doctor.
- The capsules are for oral inhalation only using the provided inhaler device. They should never be swallowed.
- Remove the capsule from the blister pack only immediately before use.
- After inhaling the medicine, rinse your mouth thoroughly with water and spit it out to prevent oral thrush.
- It is recommended to consult a doctor or respiratory specialist prior to using this inhaler for optimal results.
Uses
- Chronic Obstructive Pulmonary Disease (COPD): A progressive lung disease that includes emphysema and chronic bronchitis, causing breathing difficulties and reduced airflow.
- Chronic bronchitis: Inflammation of the bronchial tube lining that causes persistent cough and mucus production.
- Emphysema: A condition causing shortness of breath due to damage to the air sacs in the lungs.
- Maintenance therapy: Long-term prevention of COPD flare-ups and improvement of overall lung function.
- Symptom relief: Alleviating chest tightness, shortness of breath, wheezing, and cough associated with COPD.
Side Effects
- Nasopharyngitis (inflammation of nasal passages and throat)
- Headache
- Dizziness
- Upper respiratory tract infection
- Sinusitis (inflammation of the sinuses)
- Oropharyngeal pain (pain in mouth and throat)
- Dyspepsia (indigestion)
- Muscle spasms or musculoskeletal pain
- Runny or stuffy nose
- Nausea
- Dry mouth
- Chest pain
- Dental caries (with prolonged use)
- Painful and frequent urination
Interactions
- Loftair Inhaler may interact with Beta-blockers (e.g., propranolol, metoprolol), which can decrease the effectiveness of Indacaterol and may cause bronchospasm.
- Inhaler may interact with Corticosteroids, potentially increasing the risk of systemic side effects when used together.
- Inhaler may interact with other anticholinergic medications, leading to increased anticholinergic effects such as dry mouth, urinary retention, and constipation.
- Inhaler may interact with Monoamine oxidase inhibitors (MAOIs) and Tricyclic antidepressants, potentially increasing cardiovascular side effects.
- Inhaler may interact with Diuretics and Xanthine derivatives (e.g., theophylline), which can increase the risk of hypokalemia (low potassium levels).
- Inhaler may interact with Linezolid, potentially leading to increased blood pressure.
Pharmacokinetics
Pharmacokinetics of Loftair Inhaler involves the absorption of the active components through the lung tissue into systemic circulation, where they produce their therapeutic effects. Following inhalation, Indacaterol is rapidly absorbed with peak plasma concentrations achieved within 15 minutes. Glycopyrrolate is also absorbed through the lungs with onset of action within 5 minutes. Both drugs have long half-lives allowing for once-daily dosing. The medications are metabolized primarily in the liver and excreted through both renal and fecal routes.
Precautions
- Allergies: Do not use Loftair Inhaler if you are allergic to Indacaterol, Glycopyrrolate, or lactose (as the capsules contain lactose monohydrate).
- Asthma: Loftair Inhaler is not indicated for the treatment of asthma. Do not use this medication if you have asthma without consulting your doctor. This medication is specifically for COPD management only.
- Acute bronchospasm: This inhaler is not a rescue medication and should not be used for sudden breathing difficulties or acute COPD exacerbations. Always carry a rescue inhaler for emergency situations.
- Cardiovascular conditions: Use with caution if you have heart problems, irregular heartbeat, high blood pressure, or a history of heart disease, as beta-agonists may cause cardiovascular effects.
- Narrow-angle glaucoma: Use with caution as anticholinergics can worsen glaucoma symptoms. Avoid getting the medication in your eyes.
- Urinary retention: Use with caution if you have prostate problems or urinary retention issues, as anticholinergics can worsen these conditions.
- Seizure disorders: Use with caution if you have a history of seizures or epilepsy.
- Diabetes: Monitor blood sugar levels closely as beta-agonists may cause hyperglycemia.
- Thyroid disorders: Use with caution if you have hyperthyroidism.
- Kidney and liver disease: Use with caution if you have kidney or liver impairment.
- Pregnancy and breastfeeding: Exercise caution during pregnancy (Category C). Limited data available for breastfeeding mothers. Consult your doctor before using Loftair Inhaler if you’re pregnant or breastfeeding.
- Age restrictions: Not recommended for children below 18 years as safety and effectiveness have not been established in this age group.
- Proper technique: Ensure correct inhaler technique for optimal drug delivery. The capsules must only be used with the provided inhaler device and should never be swallowed.
Conclusion
Loftair Inhaler 110 Mcg + 50 Mcg is an effective medication for the long-term maintenance treatment of Chronic Obstructive Pulmonary Disease (COPD). By combining two complementary bronchodilators with different mechanisms of action, this once-daily inhaler provides sustained relief from COPD symptoms, improves lung function, reduces exacerbations, and enhances quality of life. The rapid onset and prolonged duration of action make it a convenient and effective option for COPD management. However, it is crucial to use this medication regularly as prescribed, maintain proper inhaler technique, and adhere to all precautions and safety guidelines. Always consult your physician or respiratory specialist for personalized recommendations and guidance on using Loftair Inhaler as part of a comprehensive COPD management plan that may include lifestyle modifications such as smoking cessation and avoiding environmental pollutants.
References
https://doi.org/10.2147/COPD.S55488
https://www.ncbi.nlm.nih.gov/books/NBK568878/



Ashley Martinez –
First time trying this combo. Breathing definitely improved